Carbohydrates, Lipids, and Proteins
Carbohydrates are our body’s primary energy molecules. Starch and sugars from food convert to ATP during cellular respiration and lactic acid fermentation.

Lipids comprise our cell membranes and steroid hormones. Fats are our main long-term energy storage molecules.

Genes code for proteins. Proteins are our body’s structural support (collagen), used in movement (muscles), are non-steroid hormones (insulin), and speed up chemical reactions (enzymes).

Catabolism and Anabolism
Metabolism comprises breaking down molecules and building molecules. Catabolism is the process of breaking polymers (larger molecules) into monomers (smaller molecules). Digestion is an example of catabolism. Anabolism is the process of polymerization – using monomers to build polymers. Muscle and hair growth are examples of anabolism.



Enzymes
Enzymes are catalytic proteins that speed up chemical reactions. All of the body’s chemical reactions can happen without enzymes; however, the reactions will be too slow to maintain life. Therefore, every chemical reaction in your body uses enzymes for growth, repair, and homeostasis.
So how do enzymes speed up chemical reactions?
Enzymes are catalysts that lower the activation energy of a chemical reaction.
The what?
The activation energy is the energy required to start a chemical reaction. For example, assume you had to walk two miles to get to school. There are two paths, each two-mile in length. One path is level ground, and the other is a continuous hill.
Wait. How is that possible?
Just play along.
What happens if I do not want to play along?
Really?
Really.
There’s a cookie involved.
What type of cookie.
Chocolate chip.
Okay, I’ll play.
So, which path will you expend less time and energy walking to school?
The level path.
Good. A chemical reaction with an enzyme is the level path, and a chemical reaction without an enzyme is the uphill path. Both pathways are the same length and lead to the same location, but one requires a lot more energy and time to get there.


Got it. So where’s my cookie?
Finish the chapter and then you’ll get your cookie.
That was not part of the deal!!!!

Enzymes Are Specific
Enzymes are similar to the receptor proteins on a cell’s membrane in that they have a grove, called the active site that only a specific solute can attach. The active site’s shape only allows a single solute, called a substrate, to link to it (a process called induced fit) to form an enzyme-substrate complex. For example, the active site on the enzyme sucrase can only bond with sucrose (table sugar), where sucrase will speed up sucrose’s catabolism into glucose and fructose.



Enzyme Specificity
Can the enzyme sucrase react with starch?
Nope.
Can sucrase react with proteins or lipids?
Nope and nope.
So, sucrase can only react with sucrose?
Yep. Sucrase can only react with sucrose, which is why enzymes are specific in their specialization.


Enzymes are Reusable
Enzymes speed up chemical reactions, but they are not a reactant or a product of a chemical reaction. An enzyme’s active site acts as a meeting place for the substrate, which accelerates the substrate’s catabolism or anabolism. However, the enzyme remains intact and can be a part of many chemical reactions.

Enzymes Function in Optimal Conditions
The best 100-meter sprinters spend the first 30-50 meters accelerating, and the last 30-50 meters slowing down. So, why do sprinters decelerate before they reach the finishing line? Sprinting is an anaerobic exercise; therefore, muscles rely on fermentation to convert glucose into ATP. However, a cell only nets a total of 2 ATPs during anaerobic exercises. This is nearly 18 times fewer ATPs than a cell will extract from glucose during aerobic respiration.
Also, the build-up of acid in the intracellular fluid (caused by a rise in H+ concentration) and heat released from fermentation and muscle contraction denature the active sites of enzymes needed for fermentation. Denature means that the active site loses its shape, preventing it from reacting with its substrate. When too many enzymes become denatured, the chemical reactions slow, affecting cell homeostasis. Denatured enzymes mean the muscles can no longer contract at maximal force, and the sprinter begins to slow.
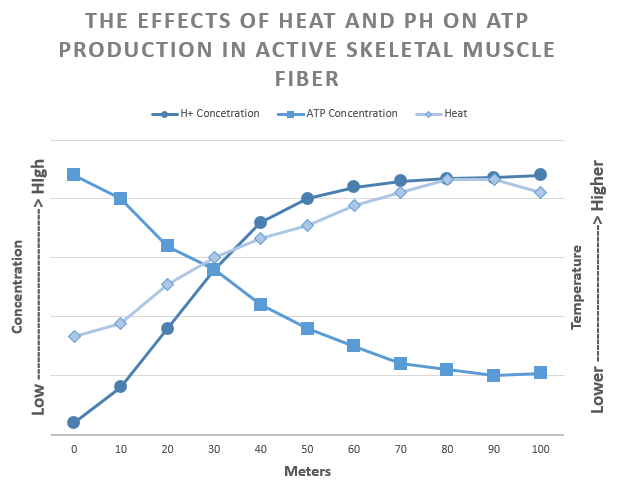
Therefore, enzymes have an optimal function in specific temperatures, pH, and salinity. For example, the digestive enzyme pepsin works at pH 2 while the digestive enzyme trypsin functions at pH 6.




Umm. . . cookie?

Did you put raisins in the cookie?!!!
Yes.
Raisins do not belong in a chocolate chip cookie!!!!!!
They do if it’s a raisin and chocolate chip cookie.
Are you evil?!!!
Yep. I am the only remaining member of the League of Evil. I think the cookies could use some sauerkraut.

AP Bio Prep
Click here for a deeper understanding on enzymes.