What is Osmosis?
Osmosis is about water. Yep, just water. More specifically, osmosis is the diffusion of water across a selectively permeable membrane, or how it moves through cell membranes.
So, we are going to watch the movie Osmosis Jones to learn about osmosis?
No.
Why not?
Because we don’t have enough time to and it really is not that accurate.
This is a picture of my water dragon, Osmosis Jones.

What? How does that relate to osmosis or the movie?
Oh!!! You can do the dumb dragon shtick in most of the chapters, but the moment I turn the table, you act like dragon facts are not inductive for learning.
Point taken.
So, we are going to watch Osmosis Jones?
No.
Fine, then here is a slide show of water dragons and alpacas, which makes about as much sense as your lessons.
Where are the arrows showing the direction of osmosis?
It’s not a literal explanation of osmosis – t’s an apt description of this class. Nix the “NGSS Biology” title, and rename it “NGSS Water Dragons and Alpacas Random Fictious Facts You Never Need to Know.”
That is a great title.

Osmosis is a Passive Process
So, water movement is only osmosis if (1) there is a semipermeable membrane separating two water solutions, and (2) the water moves passively through said membrane. If there is no membrane or if another force is pushing the water, such as active transport, then water movement is not osmosis.

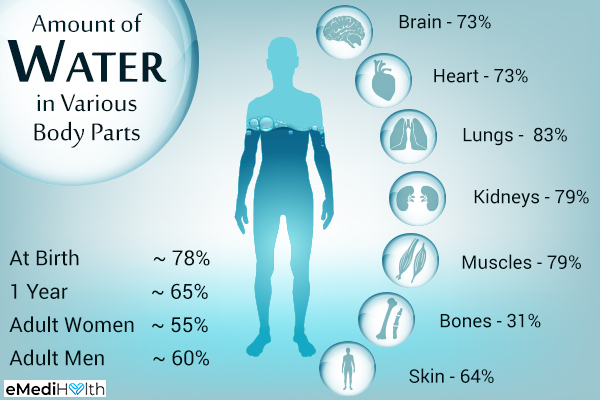
Water is the main ingredient in our body fluids and comprises 60% of our body’s mass. The water molecule also has all of these unique properties that make it the perfect essential chemical that all life needs. However, we will only focus our attention on how water moves in and out of cells, or, simply, osmosis.
Water will move to the side of a cell membrane with a higher solute concentration – this does not mean that the side with the higher solute concentration has more water than the side with fewer solutes. However, the side with a higher solute concentration will have a higher solute to water ratio than the side with fewer solutes. Therefore, water will move to the side with the higher solute concentration until the solute to water ratio is the same on both sides of the membrane (equilibrium).

For example, assume you have a box separated into equal parts by a membrane that only allows water to move through it. Each side of the box has one liter of water in it, but Side A has 10 grams of sugar, and Side B has 20 grams of sugar. Both sides have the same volume of water but different concentrations of sugar. Side B has twice as much sugar as Side A, so Side B has a higher sugar to water ratio than Side A. Remember, only water can diffuse through the membrane, so the amount of sugar on both sides of the membrane remains constant. Therefore, water will move from Side A (lower sugar concentration) to Side B (higher sugar concentration) until both sides have the same glucose and water ratio, called equilibrium.


Assume you and a friend each have a liter of water. Your friend’s glass of water has two tablespoons of sugar dissolved in it, and your glass of water has one tablespoon of sugar. Your friend says his water is too sweet, but you think your water is not sweet enough. Neither of you has access to more water or sugar, but you have a membrane that is only permeable to water. (I have no idea why you would have a selectively permeable membrane but no source of water or sugar, but please play along.) You pour your solution into your friend’s cup until both glasses have equal parts of water and sugar. Now, notice how your friend’s cup has more liquid than yours. Your friend has more sugar water than you do because you got rid of water to increase the sugar concentration, and your friend gained water to decrease the sugar concentration. Now both of you have the same concentration of sugar water but unequal volumes.
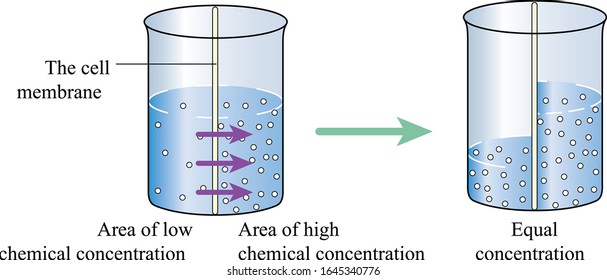
It is important to note that osmosis involves water movement towards a higher level of solutes, not to the areas with larger water volumes.
Solute Concentration Affects the Direction of Osmosis
A solution a cell is immersed in three different states.
An isotonic solution has the same solute concentration as inside the cell; therefore, it is at equilibrium. Water will move back and forth across the membrane equally.

A hypertonic solution contains more solutes than the solution inside a cell. Since the inside of the cell has a lower solute concentration, water will leave the cell.

A hypotonic solution contains fewer solutes than the solution inside a cell. Since the inside of the cell has a higher solute concentration, water will enter the cell.

AP Bio and Physiology Prep
Here is a link to my Physiology chapter on osmosis. Warning: I take it up to 11.

















